Fang Guo's Group Published in Nature Communications on Temperature and Sleep
On April 2, Fang Guo's research group at the School of Brain Science and Brain Medicine at Zhejiang University published a research paper titled "Dynamic Encoding of Temperature in the Central Circadian Circuit Coordinates Physiological Activities" in the prestigious journal "Nature Communications". Employing advanced experimental techniques, the study unveiled the pivotal temperature-sensing circadian neuron DN1a in Drosophila, capable of rhythmically responding to environmental temperature fluctuations. DN1a neurons modulate Drosophila sleep activity at varying temperatures by targeting distinct downstream circadian neurons, namely LNd and DN3. This groundbreaking discovery lays a critical experimental foundation for elucidating the mechanisms through which circadian neurons regulate animal physiological activities across different thermal environments.
Across species, including humans, circadian activity patterns typically entail activity during daylight hours and rest during the night. As seasons transition, these rhythms adapt accordingly. For instance, colder autumn and winter months often prompt earlier daytime activities due to earlier nightfall. Conversely, in hot summers, high temperatures can impede sleep onset. Moreover, the escalating greenhouse effect and global warming amplify the impact of temperature fluctuations on human sleep patterns. Consequently, comprehending the intricate interplay between temperature and the circadian mechanism of animal sleep has emerged as an imperative research focus.
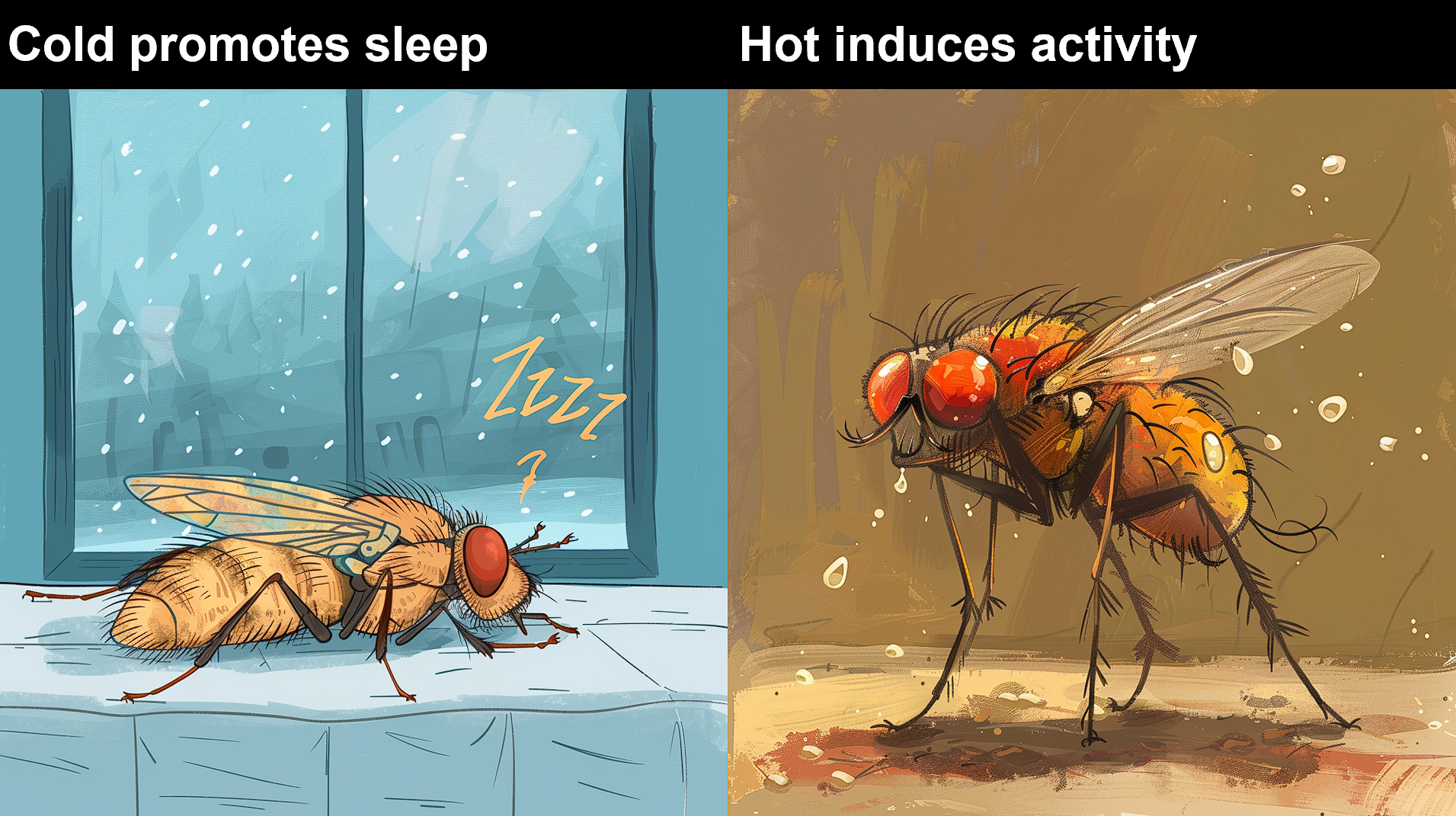
Drosophila has emerged as an exemplary model organism for dissecting circadian mechanisms owing to its distinctive physiological traits. Notably, research on the Drosophila circadian clock, which elucidated fundamental insights, was honored with the 2017 Nobel Prize in Physiology and Medicine, with Michael Rosbash among the laureates. Within the fruit fly's brain lies a neural circuit comprising approximately 75 pairs of circadian neurons, orchestrating various behaviors such as feeding, locomotion, sleep, and wakefulness at distinct times of the day. Previous investigations have underscored the role of DN1a neurons in responding to low temperatures[1, 2], while DN1p neurons are implicated in Drosophila's physiological responses to temperature fluctuations[1, 2]. Nonetheless, the identity of the pivotal regulator governing temperature regulation within the central circadian neural circuit remains an unresolved enigma.
In this study, researchers employed in-vivo two-photon calcium imaging technology coupled with a new-developed precision temperature control system. They discovered that DN1a neurons exhibit a nuanced response to temperature variations: being inhibited by low temperatures and excited by high temperatures, with a diurnal pattern of response characterized by weak and strong rhythmic changes during nighttime. Molecular mechanism investigations revealed that the temperature-dependent rhythmicity of DN1a is governed by the intrinsic circadian clock. Notably, RNAi screening unveiled the role of the circadian clock, specifically circadian proteins, in regulating calcium signal oscillations in circadian neurons via the SERCA calcium pump protein on the endoplasmic reticulum, shedding light on the circadian regulation of neuronal calcium levels. Furthermore, the study elucidated that DN1a neurons, after integrating environmental temperature cues, target distinct downstream circadian neurons—LNd and DN3—thus regulating early initiation of evening activity (DN1a-LNd) in Drosophila at low temperatures and increased nighttime activity (DN1a-DN3) at high temperatures.
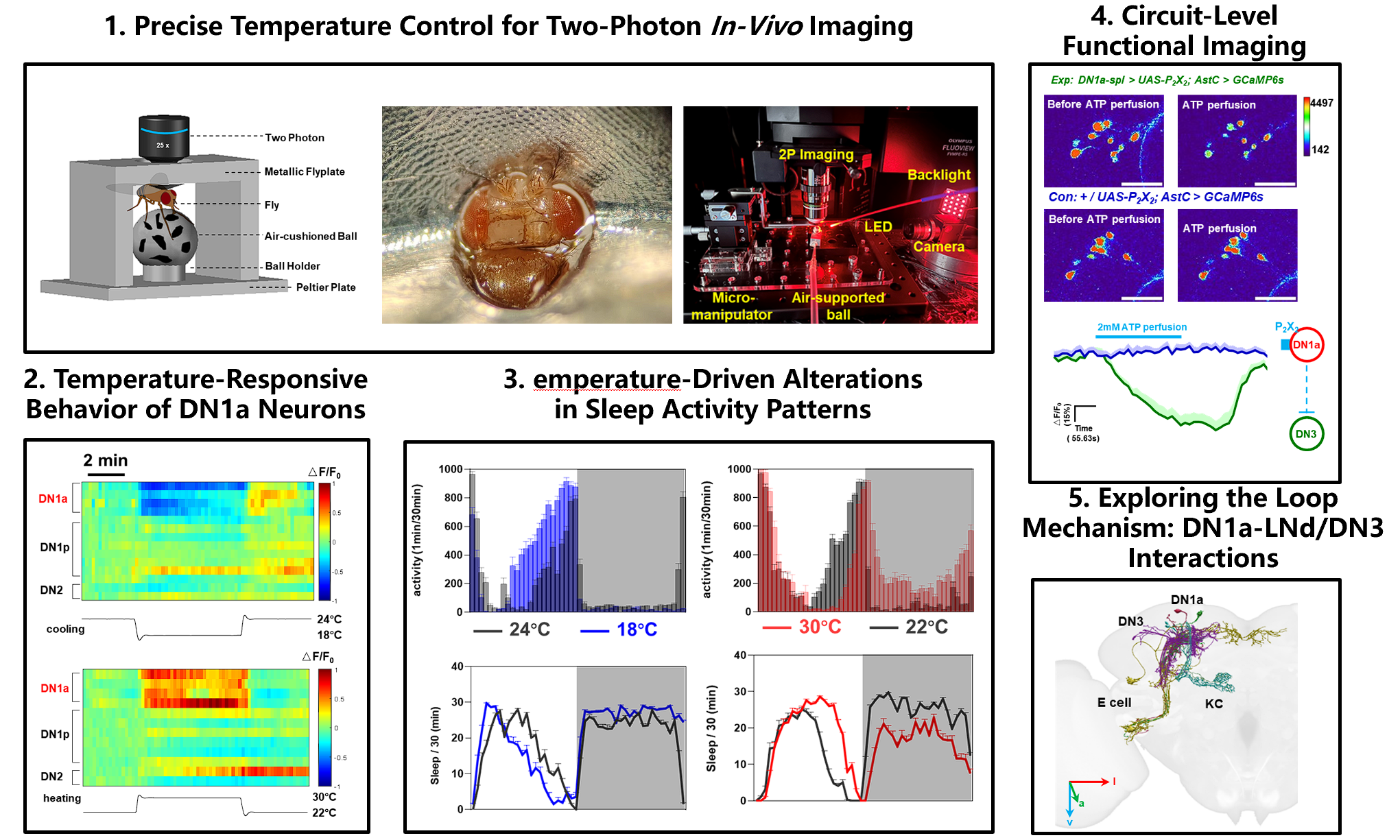
The Circuit Mechanism of Circadian Neuron DN1a in Regulating Temperature-Dependent Changes in Sleep Activity
This study offers significant insights into the influence of temperature on sleep activity, particularly amidst global warming and escalating greenhouse effects, which are altering the physiological dynamics of animals. Elevated temperatures have been associated with increased prevalence of issues such as sleep disorders and anorexia in animals, suggesting a potentially enduring impact of climate warming on the physiological activities and disease occurrence in both humans and diverse animal species [3, 4]. While the precise mechanisms underlying the effects of temperature on animal physiology remain incompletely elucidated, Drosophila, renowned as a classic model organism for circadian biology, offers a rich array of genetic tools that can aid in uncovering the physiological responses to varying temperature environments. Leveraging conserved signaling pathways and neural mechanisms underlying activity changes, in-depth exploration of circadian mechanisms in model organisms like Drosophila promises valuable insights into understanding how humans and other animals respond to environmental changes at a physiological level.
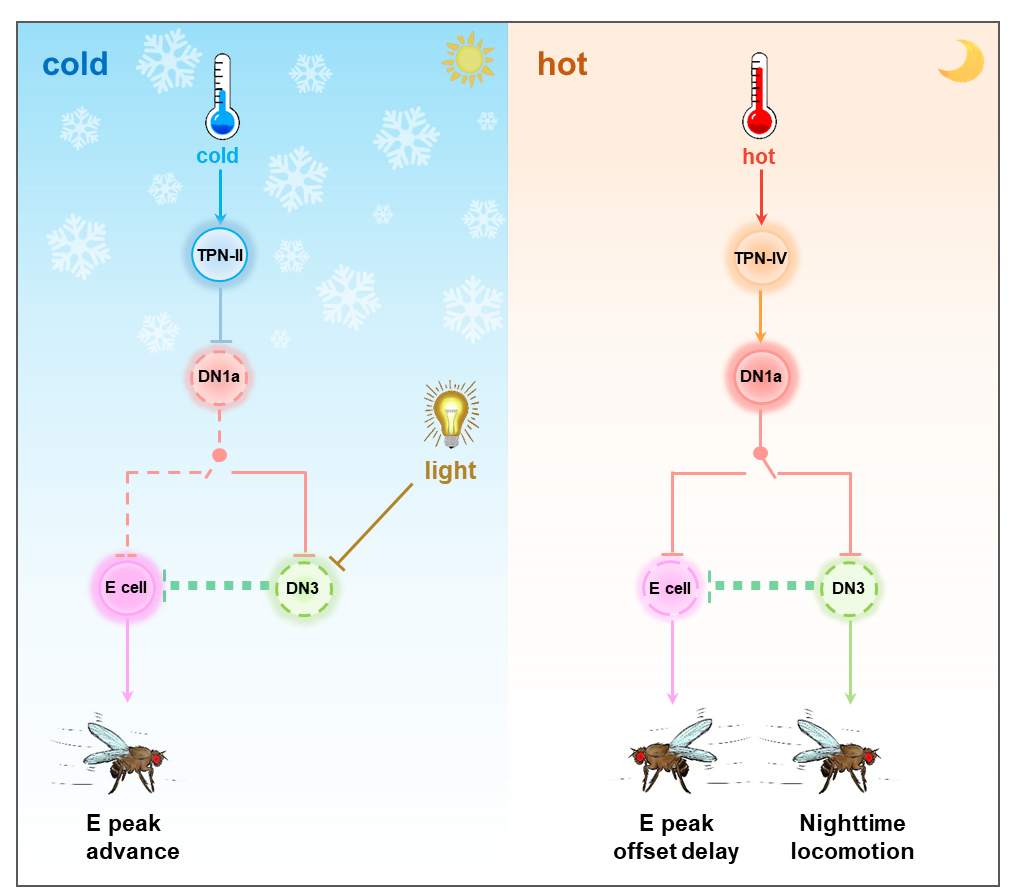
Schematic illustrating the regulation of sleep/wake patterns in Drosophila by DN1a under different temperature conditions.
Hailiang Li, a PhD student at the School of Brain Science and Brain Medicine, Zhejiang University, served as the first author of the paper, with PhD student Zhiyi Li also contributing significantly. Researcher Fang Guo acted as the corresponding author. The School of Brain Science and Brain Medicine of Zhejiang University is listed as the first author organization. The research received support from Prof. Xiaoming Li and Prof. Wei Gong from Zhejiang University, as well as Prof. Junhai Han from Southeast University. Funding for the research was provided by the Key Research and Development Program of the Ministry of Science and Technology, the National Natural Science Foundation of China, the National High-level Young Talents Program, and the Science and Technology Innovation Team 2.0 Support Program of Zhejiang University.
Dr. Fang Guo, a researcher and doctoral supervisor at Zhejiang University School of Medicine, was selected for the National High-level Young Talents Program. He returned to ZJU to establish his lab in early 2018 after completing his postdoctoral fellowship in Michael Rosbash's lab. Dr. Fang has made significant contributions to the fields of circadian rhythms and sleep regulation, publishing several impactful academic papers in journals such as Nature, Neuron (2018, 2022), Nature Communications, PNAS, and Elife. Currently, the group is seeking postdoctoral candidates, and individuals interested in the field of neurology are encouraged to contact researcher Guo Fang via email at gfang@zju.edu.cn.

1.Yadlapalli, S., et al., Circadian clock neurons constantly monitor environmental temperature to set sleep timing. Nature, 2018. 555(7694): p. 98-102.
2.Jin, X., et al., A subset of DN1p neurons integrates thermosensory inputs to promote wakefulness via CNMa signaling. Curr Biol, 2021. 31(10): p. 2075-2087.e6.
3.Siegel, J.M., Sleep function: an evolutionary perspective. Lancet Neurol, 2022. 21(10): p. 937-946.
4.Gutiérrez, E., R. Vázquez, and R.A. Boakes, Activity-based anorexia: ambient temperature has been a neglected factor. Psychon Bull Rev, 2002. 9(2): p. 239-49.







