Lijun Kang's Group Published in Neuron on Glial GABA Signaling
Glial cells play a crucial role in the development, function, and health of the nervous system and brain. From Caenorhabditis elegans, Drosophila to zebrafish, mice, and humans, the origin, structure, and function of glial cells have been highly conserved [1].
On March 6, 2024, Professor Lijun Kang and his team published a research article titled "Phasic/Tonic-like Glial GABA Differentially Transduce for Olfactory Adaptation and Neuronal Aging" in the journal Neuron. In their study, they discovered that AMsh glial cells regulate real-time olfactory adaptation and long-term neuronal aging through two distinct GABA signaling pathways (Figure 1) [2].
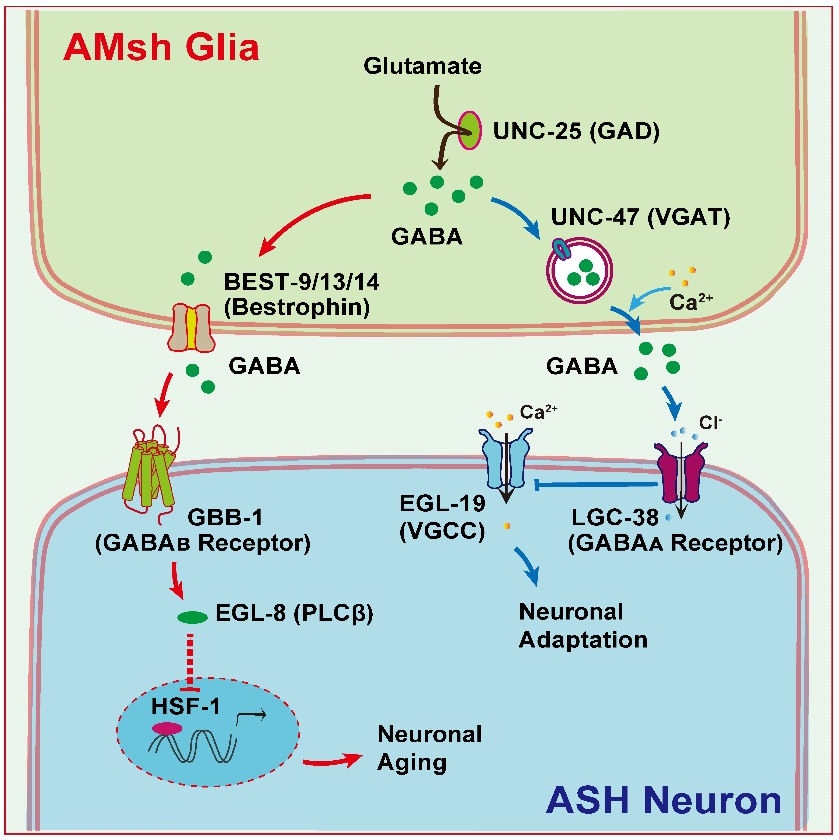
Figure 1: Differential regulation of olfactory adaptation and neuronal aging by phasic/tonic-like glial GABA signaling.
Adaptability is one of the fundamental characteristics of the nervous system. In their previous research, Professor Lijun Kang's team revealed that sheath-like glial cells called AMsh glia sense odorant stimuli via G-protein coupled receptors (GPCRs) in the chemosensory organ of C. elegans. These glial cells release GABA, which acts on the GABAA receptor LGC-38 in ASH sensory neurons, leading to the inhibition of their activity and promoting olfactory adaptation. This work proposed a dual receptor model involving glial cells and neurons for olfactory sensation, emphasizing the essential role of glial cells as driving forces behind neuronal adaptation (Neuron 2020) [3].
In their recent publication in Neuron, Professor Lijun Kang's team demonstrated that AMsh glial cells elevate cytoplasmic calcium levels upon sensing odorant stimuli. This elevation triggers the secretion of GABA from vesicles, a process dependent on the vesicular GABA transporter UNC-47/VGAT. The released GABA acts on ASH sensory neurons to facilitate olfactory adaptation. Additionally, at resting calcium levels, AMsh glial cells can gradually release GABA through bestrophin ion channels (Best-9/-13/-14), which activates the GABAB receptor GBB-1 on ASH sensory neurons, and regulates the activity of the transcription factor HSF-1 via the PLCβ signaling pathway, thus slowing down the aging process of ASH neurons.
This research has revealed two distinct GABA signaling pathways within the local circuitry involving AMsh glial cells and ASH sensory neurons. Each pathway plays crucial physiological roles: (1) UNC-47/VGAT--LGC-38/GABAA fast signaling pathway. The key gene UNC-47 is predominantly expressed in the soma and proximal processes of AMsh glial cells, while LGC-38 is localized to the soma and axons of ASH neurons. (2) Bestrophin channels--GBB-1/GABAB slow signaling pathway. The critical genes, the bestrophin channels and GBB-1, are extensively expressed in AMsh glial cells and ASH neurons, respectively. Notably, UNC-47/VGAT-dependent GABA release is triggered by high calcium levels, whereas bestrophin channels can release GABA under low calcium conditions. Furthermore, GBB-1/GABAB receptors exhibit higher affinity for GABA compared to LGC-38/GABAA receptors. These findings provide novel insights into the significant role of glial cells in sensory encoding and neuronal aging.
References:
(1) Nagai et al., Behaviorally consequential astrocytic regulation of neural circuits. Neuron (2021) 109, 576-596.
(2) Cheng et al., Phasic/Tonic-like Glial GABA Differentially Transduce for Olfactory Adaptation and Neuronal Aging. Neuron. 2024 Mar 1:S0896-6273(24)00090-4. doi: 10.1016/j.neuron.2024.02.006.
(3) Duan et al. Sensory glia detect repulsive odorant and drive olfactory adaptation. Neuron (2020) 108,707-721.







