Dr. Ying Shen/'s Team published in Journal of Neuroscience
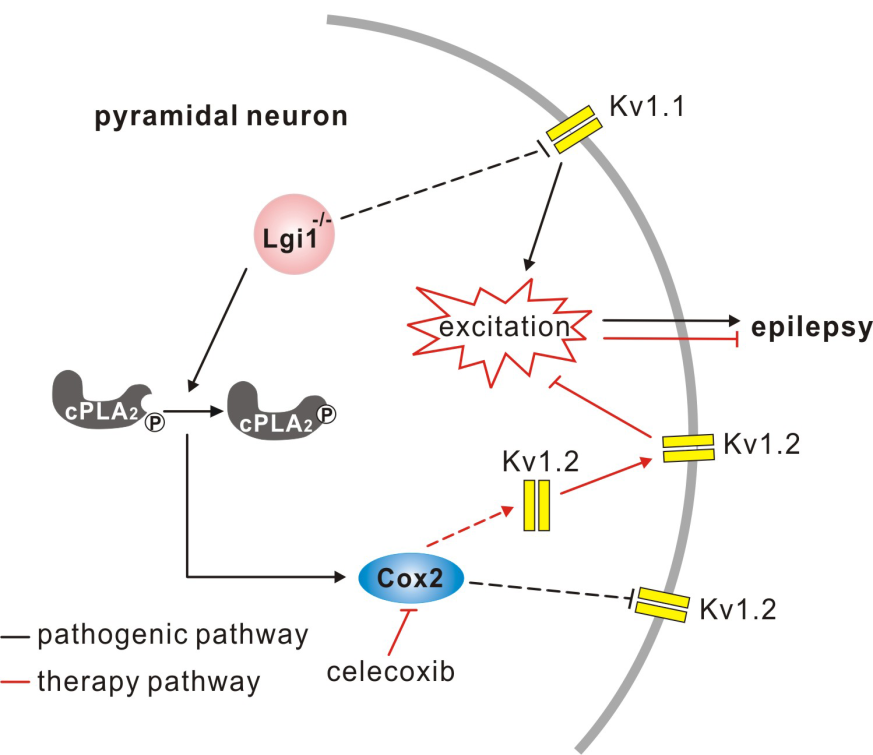
On 2/28/2018, the Journal of Neuroscience published online a research article entitled “Celecoxib Ameliorates Seizure Susceptibility in Autosomal Dominant Lateral Temporal Epilepsy” from Prof. Shen’s group. This study reveals that the cause of seizures in Autosomal Dominant Lateral Temporal Lobe Epilepsy (ADLTE) is the increased discharges of cortical pyramidal neurons, the underlying mechanism of which is the increased activity of cPLA2-Cox2 pathway and decreased Kv1.2 expression. More importantly, they discover for the first time that a drug used to treat chronic inflammation can significantly ameliorates the occurance of seizures and prolong the lifetime of ADLTE mice. This work offers a new insight about the treatment of patients with ADLTE or even refractory epilepsy.
The cure rate of epilepsy is extremely low, which brings great pain and burden to patients and their families. Because the pathogenesis is not clear, some patients cannot be cured and finally suffer from refractory epilepsy. ADLTE is such a kind of refractory epilepsy, for which carbamazepine is usually used to suppress seizures in short term. To make matters worse, carbamazepine brings patients with systemic side effects, such as hyponatremia, lupus syndrome and glandular disease. LGI1 is the pathogenic gene of ADLTE, and 34 LGI1 mutations have been identified. A Japanese group published in Nat Med (2015) to claim that 4-PBA can restore the hippocampal glutamatergic transmission, improve the symptoms of a kind of LGI1 mutant mouse, and extend its life, but is invalid for other LGI1 mutant mice or LGI1 knockout mice. Therefore, it is urgent to study the pathogenesis of ADLTE from a new perspective and find new therapeutic targets.
Using LGI1 knockout mice, Shen's group clarified that the absence of LGI1 leads to the increase of excitability of cortical pyramidal neurons, which is the cause of epileptic seizures. The absence of LGI1 reduces the expression of Kv1.2 at synapse. Kv1.2 currents decrease, meanwhile the intrinsic excitability of neurons increases, all of which lead to epileptic seizures. Many cortical pyramidal neurons in LGI1 knockout mice increase in excitability, but the excitability of interneurons does not change. This change might be caused by LGI1-cPLA2-Cox2 activity, because Cox2 activity in knockout mice increases shortly after birth and before the onset of epilepsy, indicating the impoartant difference between spontaneous seizures and hippocampus-kindeled seizures. Most importantly, Shen’s team for the first time discoveres that a few injections of celecoxib (an FDA approved drugs used to treat arthritis and menoxenia) are sufficient to restore Kv1.2 expression in pyramidal neurons, and thus reduce the excitability of neurons, reduce animal seizures, and prolong their life. Because of celecoxib has been approved for clinical medicine, it is a promising drug for the the treatment of intractable epilepsy. As a matter of fact, the treatment with the use of celecoxib and another Cox2 inhibitor has been used for a small number of refractory epileptic patients (approved by the patients) and has been greatly successful.
The study was completed by Zhou Lin (Ph.D. student), Zhou Liang (Lecturer), and so on, and was funded by NSFC. The article link: http://www.jneurosci.org/content/early/2018/02/28/JNEUROSCI.3245-17.2018







